Training Center
What is CEC and Why Is It Important?
Thursday, September 7, 2023 | Troy Buechel

We occasionally receive questions about CEC or the nutrient retention properties of our PRO-MIX products. Both questions tie into CEC. So, what is CEC?
CEC is an acronym for “cation exchange capacity” and refers to a soilless medium or soil’s capacity to hold and exchange mineral nutrients. In this article, we will talk about CEC, what it means, and its importance.
Understanding Cation-Exchange Capacity (CEC)
To understand what CEC is, we need to review a little chemistry. Conventional fertilizer comes in the form of salts. When mixed with water, these salts break apart, leaving individually charged nutrients. For example, calcium nitrate (a salt) dissociates in the water into calcium and nitrate:
Ca(NO3)2 + water = Ca++ + 2 NO3-
Both calcium and nitrate have an electrical charge. Calcium has a positive charge, or cation, and nitrate has a negative charge, or anion.
Figure 1 shows all plant elements and their charges. In the case of organic fertilizer, the same rules apply; microbes break down complex molecules into the same individual fertilizer elements as seen in Figure 1.
| Cations (positive charge) | Anions (negative charge) |
| Ammonium (HN4*) | Boron (Borate - BO3*) |
| Calcium (Ca**) | Chloride (CI*) |
| Copper (Cu*) | Nitrate (NO3*) |
| Hydrogen (H*) | Phosphate (H2PO4*) |
| Iron (Fe**) | Sulfate (SO4**) |
| Magnesium (Mg**) | |
| Manganese (Mn**) | |
| Potassium (K*) | |
| Sodium (Na*) | |
| Zinc (Zn**) |
Figure 1. When fertilizer elements dissociate in water, they are classified as either cations or anions. Because of these charges, they can be electrically bound to soilless media particles.
Negative Charge of Soil and Soilless Growing Media
Soil and soilless media particles (peat moss, vermiculite, bark, coir, calcined clay, etc.) have electrical charges on their surfaces. If there is a negative charge on the surface of a growing medium particle, it contributes to the cation exchange capacity.
Positively charged elements (cations) bind to these negative sites on the media particles and later can be exchanged for another element used by the plant. The more negatively charged sites on these particles, the higher the cation exchange capacity of the growing medium.
Positive Charge of Soil and Soilless Growing Media
Soils and soilless media particles can also have positive charges which attract negatively charged particles. These positive sites contribute to the anion exchange capacity of the growing medium. In comparison, the cation exchange capacity is more significant in a soilless media than the anion exchange capacity.
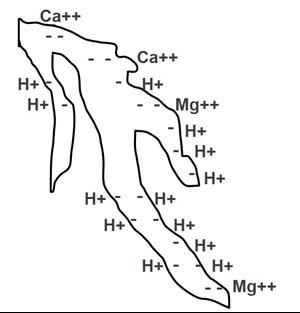
Figure 1. The peat particle pictured has negative charges that have cations attached to these sites. The number of elements retained as seen in this picture represents the cation exchange capacity of this peat particle.
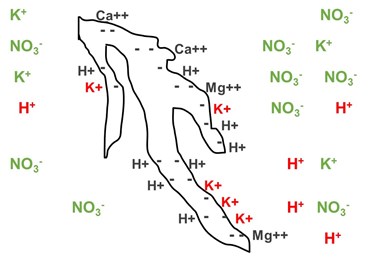
Figure 2. The same peat particle as seen in Figure 1, with potassium nitrate fertilizer added. The potassium nitrate dissociates into individual elements shown in green. Since the potassium ratio is high compared to the hydrogen (H+, or acid ions) on the peat, some of the hydrogen is exchanged for potassium (these elements are in red).
Strongest to Weakest Elements (CEC)
The binding strength of elements to these exchange sites varies depending on the type of element and its electrical charge (+1, +2, etc.). The image below shows the strongest held elements to the weakest held elements on cation exchange sites on clay particles.
Strongest -------------> Weakest
H+ > Ca++ > Mg++ > K+ = NH4+ > Na+
What this means is that calcium (Ca++) or magnesium (Mg++) is less likely to come off the exchange site than sodium (Na+), potassium (K+) or ammonium (NH4+). Therefore, since potassium is a weakly adsorbed cation and is less likely to bind to an exchange site if calcium is present, potassium is more likely to be leached from the growing medium.
Advantages of a High CEC in Horticulture
The advantage of a high CEC is not only that the soil or soilless media can hold a lot of fertilizer elements and give them back to plants later, but also it can help buffer or resist a change in pH. For example, if you add limestone to an acidic clay soil, the calcium and magnesium can displace hydrogen (acid ions) from the CEC sites.
The hydrogen bonds with the carbonate coming from the lime, causing an increase in the pH of the clay soil. The more hydrogen ions are retained by the soil, the more limestone is needed to adjust the pH of the soil.
Likewise, if fertilizer is applied to a soil, many of the fertilizer elements could bind to the CEC sites. Therefore, soils with high CEC require higher fertilizer application rates to compensate. If fertility levels decline in the soil, some of these fertilizer elements may be exchanged and become available for plant usage.
Although CEC has value in soilless media, it is not as significant when it comes to nutrient exchange and pH management as it is with the soil.
CEC Expressed on a Weight vs. Volume Basis
Cation exchange capacity is measured as milliequivalents per 100 grams of growing medium (meq/100g). However, it could also be measured as meq/100 cm3. The typical CEC values of soil and various media components are listed in Figure 1 below.
| Component / Mix | CEC (meq / 100g) |
| Fine clay | 56-63 |
| Coarse clay | 22-52 |
| Silt | 3-7 |
| Sphagnum peat | 100-120 |
| Peat humus | 200 |
| Vermiculite | 150 |
| Perlite | 1.5 |
| Aged bard | 40-60 |
| 50% Peat, 50% Vermiculite | 141 |
Figure 1. Cation exchange capacities of soil, various growing medium components, and a soilless medium.
Source: Bunt, A.C. 1988.
When looking at Figure 1, it is apparent that the CEC of peat moss and vermiculite is higher than clay. This is true on a weight basis as the CEC is expressed as meq / 100 grams. However, containers are filled based on their volume and not the weight of the growing medium they hold. Expressing CEC on a volume basis is more accurate as containerized plants only have access to nutrients within the growing medium inside the container.
Values Change on a Volume Basis
Therefore, when CEC is expressed on a volume basis, the numerical values change as seen in Table 1 below:
| Measurement | Field Soil | 1 Peat: 1 Vermiculite |
| CEC (meq/100 g) | 20 | 141 |
| Bulk Density (g/cm3) | 1.3 g/cm3 | 0.1 g /cm3 |
| Container Volume (cm3/pot) | 1250 cm3 | 1250 cm3 |
| CEC (meq/pot) | 325 | 176 |
Table 1. This table contrasts the CEC of a container filled with field soil and a 50% peat/50% vermiculite growing medium. Notice that by weight (first row), CEC is higher for the peat/vermiculite growing medium, but when compared on a volume basis (last row), the field soil has a larger CEC capacity. Source: Biernbaum, J.A. 1992.
In the first row of Table 1, the field soil with a CEC of 20 meq/100g is much lower than a CEC of 141 meq/100g for a 50% peat/50% vermiculite growing medium. This is on a weight basis. The second row of the table shows that the bulk density of the field soil is 13 times heavier than the peat/vermiculite growing medium.
If these two materials are added to individual pots of equal volume (1250 cm3), then the CEC of the field soil is 325 meq/pot, which is almost twice as high as a CEC of 176 meq/pot for the peat/vermiculite growing medium. Often, the CEC of a growing medium is lower than clay soil when expressed on a volume basis.
CEC Shrouded by Other Variables
Research from John Biernbaum and Bill Argo showed that although the CEC of a soilless growing medium had some importance in nutrient retention and in buffering against minor pH changes, other variables had a greater influence on the pH and nutrient status of the growing medium.
Limestone Charge in Growing Medium
First, the pH of the growing medium is significantly influenced by its limestone charge, the potential acidity or basicity of the fertilizer applied, and the alkalinity (carbonate and bicarbonate content) of the water. Manipulating one or all three variables has a greater influence on the long-term pH of the growing medium as opposed to the CEC.
Calcium and Magnesium Retention
Second, they found that calcium and magnesium retention by CEC sites may be considered significant in soilless media with high CEC, but not in comparison to the quantities of calcium and magnesium that need to be applied to avoid nutrient deficiencies.
In other words, unlike clay soil, which can hold enough magnesium and calcium on its exchange sites to provide them in sufficient quantities for crops throughout the growing season, soilless growing media simply cannot retain enough of these nutrients, so additional applications must be made through the crop cycle. As a result, growers do not depend on the CEC to help provide nutrients for crops grown in a soilless growing medium.
pH of Sphagnum Peat moss
Third, the pH of the growing medium influences the CEC. For example, if the pH of sphagnum peat moss increases from 3.5 to 8.0, the CEC increases by 140 meq/l. As the pH of sphagnum peat moss rises, the harder to displace hydrogen ions eventually come off cation exchange sites and allow other elements, such as calcium and magnesium, to replace them.
However, how many crops can be grown at a pH of 8.0? Typically, most crops do best when the pH of a soilless growing medium is between 5.5-6.2, so the actual CEC may be lower than published sources.
Learn more about how growing medium pH influences nutrient availability.
Supply Nutrients Through Fertilization
Although a soilless growing medium can have a reasonable CEC, it does not contribute much towards nutrient retention or prevention of pH change, unlike clay soil. If nutrients are needed, growers need to supply them through fertilization and not depend on the cation exchange sites.
Likewise, the pH of a soilless growing medium can be manipulated rather easily with limestone, a fertilizer application, and/or water alkalinity.
If you have questions about CEC and how this relates to our line of PRO-MIX and peat-based products, please contact us.
Further reading and references :
- Argo, W.R. and J.A. Biernbaum. 1997. "The effect of root media on root-zone pH, calcium, and magnesium management in containers with impatiens." J. Amer. Soc. Hort. Sci. 122(2):275-284.
- Table 1. Biernbaum, J.A. 1992. "Root-Zone Management of Greenhouse Container-Grown Crops to Control Water and Fertilizer." Hort Technology 2(1):127-132.
- Figure 1. Source: Bunt, A.C. 1988. "Media and Mixes for Container Grown Plants." Second edition. Unwin Hyman Ltd., London
If you have further questions, please contact your Premier Tech Grower Services Representative or your Regional Sales Representative.
 |
 |
 |
 |
|---|---|---|---|
|
Ed Bloodnick |
Nathan Wallace-Springer |
Lance Lawson |
Victor Brantly |
 |
 |
 |
|
|
Troy Buechel |
Susan Parent |
Jose Chen Lopez |
PRO-MIX® is a registered trademark of PREMIER HORTICULTURE Ltd.
Related Articles
-
Greenhouse Herb and Vegetable Production – Part 1/4 - Location
In this four-part series, we will look at the ideal location, structures and environment needed to grow vegetables and herbs in a greenhouse production facility. In this first part, we will discuss the first step: finding the ideal location to start a greenhouse production facility.
-
Greenhouse Herb and Vegetable Production – Part 2/4 – Greenhouse Structure
In this second part of our four-part series on growing vegetables and herbs in the greenhouse, we will focus on the greenhouse structures needed to produce quality crops.
-
Greenhouse Herb and Vegetable Production – Part 3/4 – Greenhouse Environment
In this third part of our series on growing greenhouse vegetables and herbs we will focus on the ideal greenhouse environment.
-
Greenhouse Herb and Vegetable Production – Part 4/4 – Growing Media
In this fourth and last part of our series on growing greenhouse vegetables and herbs, we will address the appropriate growing medium to use.

 Where to find our products
Where to find our products





